Part 1: Baroreceptor Response
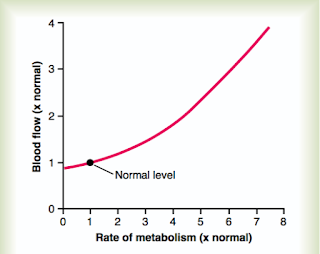 |
Figure 1: The local rate of metabolism can have a profound
effect on local arterial flow do the a local decrease in vascular
resistance. From Guyton and Hall (2016)
|
To a certain extent, the autonomic response to exercise can be thought of as an extension of the same baroreceptor response that we discussed in reference to orthostasis. In the orthostatic setting a positional drop in stroke volume resulted in decreased arterial blood pressures which stimulated the baroreceptor response. In the exercise setting, the initial drop in blood pressure is related to an exercise-induced drop in vascular resistance related to metabolic byproducts.
During muscle activity, which may range from walking to the refrigerator to running a marathon, the muscles being activated can increase their metabolic activity by 50-fold or more. In these settings of increased metabolic demand, vasodilatory metabolites are formed which include protons, potassium, phosphate, AMP, and adenosine. These substance act locally on the vascular smooth muscle which results in vasodilation.
The vasodilation is certainly intended to increase oxygen delivery to the muscle to meet metabolic demand, but it also has the effect of reducing systemic vascular resistance (SVR). Because the mean arterial pressure (MAP) is equal to the cardiac output (CO) multiplied by the vascular resistance, this local vasodilation results in a decrease in MAP. This decrease in MAP then stimulates the baroreceptor reflex which results in modulation of sympathetic and parasympathetic tone to maintain target perfusion pressures.
MAP = CO * SVR
The increased sympathetic tone carried through local sympathetic nerves result in peripheral vasoconstriction. The intention of this reflex is to increase SVR to maintain perfusing pressure in the exercising muscle. However, the mechanism of increase vascular resistance may well backfire if the sympathetic nerves also increased vascular tone at the arterioles perfusing the metabolically active tissue. For this reason, local metabolic vasodilation takes precedence over the autonomic tone and the arterial supply to the metabolically active tissue remains vasodilated. The net result of this balance between metabolic and autonomic hemodynamic homeostasis is that the exercising muscle is perfused at the expense of other non-metabolically active tissues and that the MAP remains elevated for supply of vital organs such as the heart and brain.
Part 2: The exercise-pressor response (EPR)
The sensors in place are not completely understood, but seem to consist of diverse metabolic and mechanoreceptors generally termed "ergoreceptors". Signal from these sensory neurons are carried to the lumbar dorsal horn of the spinal cord by thinly myelinated (group III) and unmyelinated fibers (group IV "C-fibers"). This pathway terminates on the NTS similar to the baroreceptor fibers, but exerts and inhibitory, rather than excitatory role. The net result of this is to inhibit parasympathetic tone and amplify sympathetic tone. Along with this, the effect of inhibiting the excitatory inputs to the NTS results in forcing the baroreceptor reflex to operate at a higher MAP.
Part 3: The role of Central Command
Continuing with our earlier line of thought, while EPR is superior to the baroreceptor response to avoid transient hypoperfusion during early exercise, it still does require increased metabolic activity to function. The ideal homeostatic mechanism for exercise would be one that initiated tachycardia and hypertension prior to any increased metabolic activity, so that the appropriate hemodynamic reserve would already be in place once exercise had begun. This mechanism is called central command.
Because central command takes its cue from cortical centers of higher-level processing which we do not understand very well, the physiology of central command is somewhat murky. At a basic level, central command is a direct inhibitory signal to the NTS which comes proximally from the paraventricular nucleus (PVN), but reflects the integration of multiple higher-level processes.
The net result is that during moderate exercise, higher level processing causes an increase in the sympathetic/parasympathetic ratio in order to prepare for exercise. Once exercise has begun, central command and EPR adjust the baroreceptor reflex to operate at higher mean pressures. At these higher pressures, second-to-second hemodynamic fluctuations are managed by the baroreceptor reflex to keep the MAP at a constant appropriate level relative to the degree of central activation and peripheral exercise intensity.
References:
Guyton and Hall Textbook of Medical Physiology (2016)
Michellini et al 2015
The net result is that during moderate exercise, higher level processing causes an increase in the sympathetic/parasympathetic ratio in order to prepare for exercise. Once exercise has begun, central command and EPR adjust the baroreceptor reflex to operate at higher mean pressures. At these higher pressures, second-to-second hemodynamic fluctuations are managed by the baroreceptor reflex to keep the MAP at a constant appropriate level relative to the degree of central activation and peripheral exercise intensity.
References:
Guyton and Hall Textbook of Medical Physiology (2016)
Michellini et al 2015










